A nationwide UCLA study found that gay and bisexual men became more aware of PrEP — a regimen that is highly effective at preventing HIV — between 2016 and 2018, but the use of PrEP among those men remained low and inconsistent during that time period.
“We are heartened to see an increase in PrEP familiarity in this relatively short period of time,” Ian Holloway, an associate professor at UCLA’s Luskin School of Public Affairs and the study’s lead author, said in a statement. “But growth in favorable attitudes was modest, as was the increase in PrEP use among sexually-active gay and bisexual men.”
The study surveyed men who were 18 to 25, 34 to 41, and 52 to 59 in three waves between March of 2016 and March of 2018 on their familiarity with and attitude towards PrEP and their use and discontinued use of PrEP over that time span.
White men accounted for 63 to 66 percent of the sample, Black men accounted for 13 to 15 percent of the sample, and Latino men accounted for 21 to 22 percent of the sample.
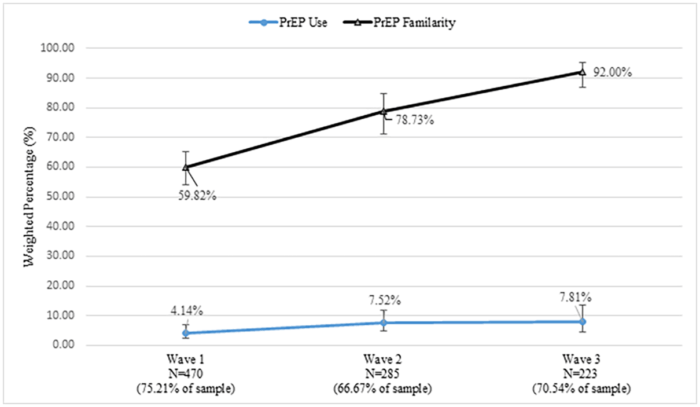
In 2016, 60 percent of the men reported “familiarity” with PrEP, but that measure climbed to 92 percent by the third wave of the study. Favorable attitudes toward PrEP went from 68 percent to 73 percent over the course of three waves. PrEP use was at four percent in 2016 and rose to eight percent in 2018, marking is a significant increase, but still represented a small number of users. Among men who reported PrEP use in the first and second waves, 33 percent reported they had stopped using PrEP in a later survey.
“It’s concerning that PrEP uptake remains low and that a third of men discontinue PrEP,” Holloway said in the statement.
Currently, there are two drugs, Truvada and Descovy, that are approved for use as PrEP. Both are manufactured and marketed by Gilead Sciences, a pharmaceutical company, and are also used to treat people who are HIV-positive. The PrEP regimen requires that users take a single pill once a day, but the drugs can also be used episodically. They are highly effective at preventing HIV infections when taken correctly.

Truvada was approved by the federal Food and Drug Administration in 2012. It was seen as breakthrough in HIV prevention efforts that previously relied on pro-condom messaging and sometimes programs that resembled group therapy that encouraged men to discuss obstacles they experienced in using condoms. It was difficult to measure the success of those efforts. Descovy was approved in 2019.
Truvada was to be a linchpin in various efforts to end the AIDS epidemic in the US that began in 2013, 2014, and 2015. The widespread use of Truvada, combined with getting larger numbers of HIV-positive people on anti-HIV drugs so they could not infect others, was going to drive down the annual number of new HIV infections in the US to the point where every newly infected person was infecting less than one other person on average. That would mean the epidemic would eventually end.
While state and local health departments and AIDS groups across the country have been successful in moving HIV-positive people into effective treatment, Truvada uptake has never reached the numbers needed to end the epidemic. That is due in part to anti-PrEP messaging from some parts of the community, notably the AIDS Healthcare Foundation in California, and from some law firms that specialize in class action lawsuits suing Gilead over Truvada side effects. While those lawsuits generally recruit HIV-positive people, the recruitment ads are read by possible Truvada for PrEP users as well. But other possible PrEP users may also resist taking a medication for HIV over stigma issues or concerns for side effects.
While PrEP uptake has been better among white gay and bisexual men, uptake among Black and Latino men has been slower due, in part, to fewer sustained public health efforts to engage those men.
Newer forms of PrEP that are in development may address some of the issues with the prevention mode. An injectable form of PrEP that is as effective as the daily pill, but is given once every other month, could be more popular. Among women as well as some transgender men and non-binary folks, a vaginal ring that lasts for a month is also in development.
“With new and innovative PrEP modalities like long-acting injectable PrEP coming online, we still need to know more about what factors are key to decision-making around starting and stopping PrEP,” Holloway said.
To sign up for the Gay City News email newsletter, visit gaycitynews.com/newsletter.